di ALEXANDER PURCELL
 |
The olives trees near Speccia, Italy are dead or have symptoms of Olive Quick Decline Syndrome, 2020. (Photo by Donato Boschia, with permission.) |
Environmental requirements for endemic populations of X. fastidiosa
Xylellas in plants are restricted to the host plant’s xylem - a system of cells that transport water from the roots to tissues throughout the plant. Xylem sap has a relatively simple chemical profile compared to other plant fluids. It is mostly water containing dilute solutions of chemicals. The main nutrients for xylem sap-feeders are amino acids, organic acids, along with dissolved minerals and trace amounts of proteins, sugars and other organic molecules9-10. Special microbes that only inhabit xylem are not commonly found in most plants, but this view may be incorrect because we direct most of our microbial investigations of xylem emphasize microbes that cause disease.
The “disease triangle” of pathogen-plant-environment serves as a classic model for plant disease epidemiology. For disease caused by X. fastidiosa, the “environment” factor includes the insect vectors required to move this pathogenic bacterium from plant to plant. Infection alone is not the same as disease and does not inevitably lead to disease, as X. fastidiosa subsp. fastidiosa illustrates very well¹¹. As already mentioned, X. fastidiosa usually does not cause observable symptoms in the majority of the plant species that it infects. In addition, many infected but symptomless plants recover from infection over time for unknown reasons6-8. Some infections disappeared from grapevines either without or after symptoms appeared after freezing exposures in a laboratory setting¹¹ or outdoors during winter12-13. The severity of winter subfreezing temperatures seems to coincide with the incidence of Pierce’s disease in North America, suggesting that winter freezing temperature regimes limit the geographical range of Pierce’s disease of grapevines12-14. Either the appearance of disease symptoms associated with diseases caused by xylellas or by detection methods for this bacterium have rarely occurred in severe winter areas. For example, molecular testing of elms with marginal leaf necrosis (leaf scorch) in southern Ontario, Canada detected X. fastidiosa in 3 of 114 trees15, suggesting that this disease occurred but was rare in this cold winter region. The resistance or tolerance of all wild grape species indigenous to the southeastern United States led Hewitt16 to conclude that this region of the USA was the probable region of origin for Pierce’s disease brought to California on wild grapes from the southeastern states for use as rootstocks.
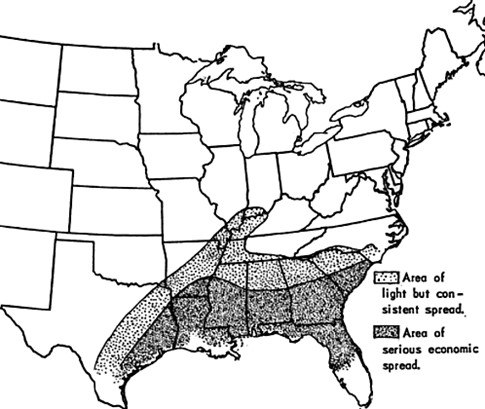
Phony peach disease, caused by X. fastidiosa subsp. multiplex, is another example that supports the hypothesis that winter cold severity limited outdoor transmission tests with insect vectors and also represents a new encounter disease. In the case of phony peach disease, both the peach (Asian origin) and the xylella genotype (as inferred from is first observance and consequent spread) were exotic to where they first occurred. Outdoor vector transmission experiments in Chattanooga, Tennessee were inconclusive because of the lack of expected symptoms, but similar outdoor cage tests later conducted in Georgia succeeded in transmission17, which is consistent with winter climate limiting the winter survival of X. fastidiosa, symptom expression, or both. Peach is an introduced plant in North America, and was well established by the mid-1800s. The first record of phony disease was in central Georgia in about 1885, afterwards expanding from this initial sighting until it stabilized in the early 1930s18 at its current geographic distribution18. The documented spread of phony disease has all the features expected of a new encounter disease. The stabilization of the geographic limits of the disease in the 1930s with little change since further supports the hypothesis that winter cold severity is a limiting factor as to where the disease can be endemic.
 |
Maps of the estimated distributions of phony disease of peach in 1928 (top) and 1933. (From Hutchins, 1935.) |
The genetic differences among the species and subspecies of the genus Xylella vary enormously as to what host plants develop disease. Subspecies of X. fastidiosa are delineated according to the similarity of DNA sequences in their genomes19. One of the first examples to document some effects of genetic variability on plant disease expression showed that a genetic grouping of isolates of X. fastidiosa subsp. fastidiosa from diseased grape and almond plants all caused disease in grape, but none of the genetically grouped isolates of the bacterium included in the subspecies multiplex from almond caused disease in grape. All the isolates from two almond genetic groupings infected grapes without causing symptoms20. The genetic uniformity of isolates of X. fastidiosa subspecies (subsp.) pauca from olive with OQDS, as well as disease in other plants hosts3, supports the hypothesis that a single introduction of one genotype of X. fastidiosa triggered the current OQDS epidemic in olive trees in Puglia. Slightly different genotypes in the same subspecies pauca cause disease in orange and coffee in Brazil, but the genotype that causes OQDS in Italy does not infect citrus or grape21. Experiments in Brazil showed that very closely related genotypes of X. fastidiosa from diseased coffee neither infect nor cause disease in orange; genotypes that cause disease in orange do not infect coffee22. In summary, small changes in the genomes of X. fastidiosa can cause major differences in the range of plant species that the bacterium can infect and which plant species develop disease symptoms.
Other examples of new encounters
Plants introduced by Europeans in their colonization of the Americas often produced larger crops than they produced in Europe because they were not accompanied by diseases and pests native to Europe or Asia, but other imported crops did not survive in their new environment. One notable example of an introduced crop plant from the Old World (Eurasia) encountering a native pathogen in the New World (Western Hemisphere) is that of Xylella fastidiosa and the commercial grape of Eurasia, Vitis vinifera.
Insect vectors of X. fastidiosa
Sucking insects that specialize in feeding on xylem sap should be considered as potential vectors of X. fastidiosa310. Europe is fortunate in having only one native species (Cicadella viridis) in a subfamily (Cicadellinae) of leafhoppers (family Cicadellidae) that specialize in feeding on xylem sap. A few other species of leafhoppers in this subfamily of xylem sap-feeders invaded Europe accidentally from North America, probably as eggs embedded within imported plants or as nymphs on live plants. Fortunately, these invasive sharpshooters have not been found feeding on plant species so far shown to be disease hosts of X. fastidiosa. Some species in other insect families of xylem sap-feeding insects are common and often abundant in Europe. All spittlebug species (superfamily Cercopoidea) specialize in feeding on xylem sap, and all tested species have proven to be X. fastidiosa vectors31.
The European species of spittlebug Philaenus spumarius is the most important vector for spreading OQDS in olives in the Salento region of Puglia in Italy
32-33. In the region severely affected by OQDS P. spumarius can be extremely abundant in weeds in olive orchards; from 10 to over 100 nymphs of P. spumarius per square meter would not be unusual. Adults move to olive trees after weeds decline in summer dry periods (May-August), and over 50% of the spittlebugs collected from olive trees tested positive for the presence of X. fastidiosa in late summer or autumn32. The lowest number of bacteria required for the insect to infect a plant by feeding is below the limit for detection tests (typically about 100 bacteria per sampled head), so actual percentage of infective spittlebugs can be even higher than estimated by the most sensitive methods35-36. P. spumarius transmits to olive in lab tests within the range of about 5 to 15% per insect per day. Without complicated math, you can easily imagine the difficulty of protecting large trees for many years from a single infection by an infectious spittlebug. At least a few spittlebugs will survive insecticide treatments, and others may fly in from adjacent untreated areas.
In all Mediterranean regions, many millions of spittlebugs every year for centuries have fed on olives with no significant adverse effects on olive production. But the introduction of a genotype of X. fastidiosa that can infect and kill an olive tree radically changed the status of P. spumarius36. Reducing the number of spittlebugs capable of transmitting X. fastidiosa to a very low number should be the objective of methods to reduce the chance of infecting a tree. The exponential rate at which OQDS spreads within an olive orchard point to olive as the main source from which spittlebugs acquire X. fastidiosa. The CVC disease in Brazilian citrus has the same exponential spread, and the regular inspection of citrus orchards followed by removing symptomatic trees greatly reduces the spread of CVC37. The similarities between CVC and OQDS reinforces the logical conclusion that removing diseased trees is an essential part of slowing the spread of OQDS. However, the pruning methods for control of CVC in Brazilian citrus does not offer much hope that pruning alone will be of benefit to reduce the spread of OQDS. In summary, a combination of control techniques against the vector and the rapid removal of infected trees has the best chance of slowing the spread of OQDS. The most effective remedy for managing OQDS will be OQDS-resistant varieties or effective new preventive or curative treatment methods that may be developed in the future.
Invasive insect vectors of X. fastidiosa pose anther threat to European agriculture and forests. The tropical and subtropical Americas have a huge diversity of xylem sap-feeders, both spittlebugs and sharpshooter leafhoppers. Most leafhoppers insert their eggs into plant tissues; spittlebugs commonly glue their eggs to living or dead plants but also soil. Importing plants that have vectors’ eggs provides an entry to invade new territories. The sharpshooter Homalodisca vitripennis (glassy-winged sharpshooter) caused a major panic for wine grape growers in southern California after 199838. This insect is native to the southeastern United states and northeast Mexico but was not seen in California until the early 1990s. Until then, Pierce’s disease of gape was a minor problem except for a few vineyards located near habitats of native sharpshooters. The Temecula Valley is a patchwork of its two major crops: citrus and grapes. This set the scene for a major problem with Pierce’s disease when the glassy-winged sharpshooter entered Temecula Valley and began to increase to very high populations in citrus groves then moved to nearby vineyards in spring months39. The exponential increase in Pierce’s disease eliminated some vineyards within three years.
 |
|
Adult glassy-winged sharpshooter, Homalodisca vitripennis. [Editor: I suggest putting the photo of the adult Homalodisca as an inset at an upper part of the photo of the barren vineyard.] |
The severity of this new problem with Pierces disease in Temecula Valley alerted all California grape growers as well as farmers in other crops like almonds that were also sensitive to diseases caused by to the new threat created by the invasion of glassy-winged sharpshooter. Beginning in 2000, grower organizations, along with the state and federal departments of agriculture, coordinated responses to control Pierce’s disease in Temecula by treating citrus just prior to grapes producing new foliage. A federal agency (APHIS) provided funding the insecticide applications. The citrus growers did not have to pay for the insecticide treatments, but the treatments complicated and raised the costs of controlling citrus pests by methods that minimized insecticide use. This was a generous move by citrus farmers to help their neighbors. Combinations of grape grower mandatory assessments and state and federal funding supported over five million dollars per year for research to develop control methods to prevent and cure Pierce’s disease. These research efforts have begun to produce results that will begin to return profits from these investments (2014). New grape varieties resistant to Pierce’s disease have are beginning to be released40.
Needs for more knowledge of the genetic diversity of xylellas
Analyzing examples from diseases caused by xylellas provides some ideas on how to avoid further invasions by their many genetic variants (genotypes). We should expect that Olive Quick Decline Syndrome will not be the last new disease to suddenly appear because of a new combination of a unique xylella genotype and a different plant host. Pierce’s disease was been reported to occur in Kosovo in in the 1990s41, but only in 2018 was it found in Mallorca42. The eventual appearance of Pierce’s disease in Europe was considered to be inevitable and required advance plans to deal with it when it became established in new regions43. Better management of OQDS would have benefitted from planning in advance to detect new xylella diseases early and to begin control measures. This will have to be adapted not only to different countries but to different regions within countries.
The current pandemic of the COVID-19 virus is best explained as an invasion of the COVID-19 virus originating from an animal source in China44. Animal viruses are quite diverse in wild and domestic animals. Some of these viruses are the origins of “new encounters” for human virus diseases like COVID-19. Virologists search wild animals for as yet undescribed viruses that may be transmissible from human to human. This enables advance research on viruses that may become a problem in humans or domestic animals. By analogy, why don’t we do this for xylellas? Central America appears to be the “El Dorado” (the fabled city of gold sought by early Spanish explorers of the Americas) of xylellas, based on this region’s high diversity and common presence of X. fastidiosa *. Because of quarantine restrictions, research on most xylella genotypes can only be done with live bacteria in regions in which a particular genotype has been proven to occur. Two locations have both the likelihood that they have a variety of unknown xylella genotypes embedded in their territory and the scientific facilities to accommodate the needed research. Brazil hosts genotypes within the subspecies pauca, while Costa Rica harbors genotypes grouped in the subspecies fastidiosa, multiplex, and pauca. Costa Rica and Brazil are suitable choices for searches to identify genetic diversity in X. fastidiosa and evaluate the impacts of diverse xylella genotypes on plants of economic or ecological concerns. Both Costa Rica and Brazil are adjacent to other countries that may be accessible for shared research. The costs of this kind of research need not be expensive relative to the value of investing in gaining knowledge of potential threats of possible “new encounters” of now unknown genotypes that inhabit the American tropics. For example, experimental plantings of kiwi fruit plants in a variety of locations in Central and South America might reveal a Xylella genotype that causes disease in kiwi.
We should expect that locations with a high diversity of Xylella genotypes and also a great diversity of plant and vector species would also harbor a diversity of other microbes that can be antagonists to X. fastidiosa. Research with microbial antagonists have the potential to manage Pierce’s disease of grape with bacteriophages (viruses of bacteria)45 or other bacteria that inhabit xylem and aid infected plants in recovering from xylella infections before causing disease46 . Science has so far concentrated on bacteria associated with plant disease, so our scientific knowledge of the microbiota of xylem is shamefully sparse. Some unpredictable findings from basic searches may have the potential to develop into methods to prevent or manage the “new encounter diseases” caused by xylellas.
REFERENCES
1. Impact of Xylella fastidiosa subspecies pauca in European olives.. https://www.pnas.org/content/117/17/9250 or https://doi.org/10.1073/pnas.1912206117
2. Hopkins, D. L. and A H. Purcell. 2002. Xylella fastidiosa: Cause of Pierce’s disease of grapevine and other emergent diseases. Plant Dis. 86:1056-1066.
3. Almeida, R. P. P. and L. Nunney. 2015. How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis. 1457-1467. [This paper also points out many examples of new encounter occurrences of X. fastidiosa.]
4. Freitag, J. H. 1951. Host range of Pierce’s disease virus as determined by insect transmission. Phytopathology 41:920-934. [In 1951 Pierce’s disease was thought to be caused by a virus and insect or graft transmission were the only methods of infecting plants in the laboratory.]
5. Hill, B. L. and A. H. Purcell. 1996. Multiplication and movement of Xylella fastidiosa in grape and four other plants. Phytopathology 85:1368-1372.
6. Purcell, A. H., and S. R. Saunders. 1999. Fate of Pierce’s disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 83:825-830. [The first evidence that symptomless hosts often recover from infection by X. fastidiosa. Recovery was also noted in Ref. 6 with many annual plants.]
7. Lopes, S. A., S. Marcussi, S. C. Z. Torres, V. Souza, C. Fagan, S. C. França, N.G. Fernandes, and J. R. S. Lopes. 2003. Weeds as alternative hosts of the citrus, coffee, and plum strains of Xylella fastidiosa in Brazil. Plant Dis. 87:544-549.
8. Wistrom, C., and Purcell, A. H. 2005. The fate of Xylella fastidiosa in vineyard weeds and other alternate hosts in California. Plant Dis. 89:994-999.
9. Andersen P. C., B. V. Brodbeck, R. F. Mizell. 1989. Diurnal and temporal changes in the chemical profile of xylem exudate from Vitis rotundifolia. Physiol. Plant. 75:63–70. [Xylem sap has a very dilute concentration of nutrients, so bacteria that live only in xylem have special adaptations, as do the sucking insects that fed mostly on xylem sap.]
10. Raven, J. A. 1984. Phytophages of xylem and phloem: a comparison of animal and plant sap-feeders. Adv. Ecol. Res. 13:135–234.
11. Purcell, AH. 2013. Paradigms: examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013. 51:339–56.
12. Purcell, AH.1977. Cold therapy of Pierce's disease of grapevines. Plant Disease
13. Purcell, AH. 1980. Environmental therapy for Pierce's disease of grapevines. Plant Disease 64:388-390.
14. Lieth, J. H., M. M. Meyer, K.-H.Yeo, and , B. C. Kirkpatrick. 2011. Modeling cold curing of Pierce’s disease in Vitis vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ grapevines in California. Phytopathology 101: 1492-1500.
15. Goodwin, P.H., and S. Zhang. 1997. Distribution of Xylella fastidiosa in southern Ontario as determined by polymerase chain reaction. Can. J. Plant Pathol. 19:13-18.
16. Hewitt, W. B. 1953. The probable home of Pierce’s disease. Plant Dis. 94-98.
17. Turner, W. F. and H. N. Pollard. 1959. Insect transmission of phony peach disease. U.S. Dept. Agric. Tech. Bull. 1193:1–27.
18. Hutchins, L. M. 1933. Identification and control of the phony disease of the peach. Georgia State Bulletin No. 78. 56 pages.
19. Schaad, N. W., E. Postnikova, G. Lacy, M. Fatmi, and C. J. Chang. 2004. Xylella fastidiosa subspecies: X. fastidiosa subsp. piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov. Syst. Appl. Microbiol. 27:290-300.
20. Hendson, M., A. H. Purcell, D, Chen, C. Smart, M. Guilhabert, and B. C. Kirkpatrick. 2001. Genetic diversity of Pierce’s diseases strains and other pathotypes of Xylella fastidiosa. Appl. Environ. Microbiol. 67:895-903.
21. Saponari, M., Loconsole, G., Cornara, D., Yokomi, R. K., Stradis, A., Boscia, D., Bosco, D., Martelli, G. P., Krugner, R., and Porcelli, F. 2014. Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 107:1316-1319.
22. Almeida, R. P. P., Nascimento, F. E., Chau, J., Prado, S. S., Tsai, C.-W., Lopes, S. A., and Lopes, J. R. S. 2008. Genetic structure and biology of Xylella fastidiosa strains causing disease in citrus and coffee in Brazil. Appl. Environ. Microbiol. 74:3690-3701.
23. Davis, M. J., A. H. Purcell, and S. V. Thompson. 1978. Pierce's Disease of Grapevines: Isolation of the Causal Bacterium. Science 199:75-77.
24. Bettiga, L. J. (editor). 2013. Grape Pest Management, 3rd edition. University of California, Agriculture and Natural Resources, Communication Services, Richmond, California.
25. K. Pednaeult and C. Provost. Fungus resistant grape varieties as a suitable alternative for organic wine production: Benefits, limits, and challenges. Scientia Horticulturae 308:57-77.
26. Goheen, A. C. 1989. Virus Diseases and Grapevine Selection. Am. J. Enol. Vitic. 40:67-71.
27. R. Van Driesche, M. Hoddle, and T. Center (editors). 2008. Control of Pest and Weeds by Natural Enemies. An Introduction to Biological Control. Blackwell Publishing, Oxford, U. K. [This is but one of many sources to describe the basic workings of biological control.]
28. Nentwig, W. (ed.). 2011. Biological Invasions. Ecological Studies 193. Springer-Verlag, Berlin. [This is only one of many choices of books on biological invasions.]
29. International Symposium on Xylella fastidiosa. 2017. Brisbane Australia. https://www.agriculture.gov.au/pests-diseases-weeds/plant/xylella/international-symposium-xylella-fastidiosa [videos of the symposium presentations on X. fastidiosa are available on Internet.]
30. European Union web site for Xylella: https://ec.europa.eu/food/plant/plant_health_biosecurity/legislation/emergency_measures/xylella-fastidiosa/latest-developments_en
31. Redak R., A. H. Purcell, J. R. S. Lopes, M. Blua, R. F. Mizell, and P. C. Andersen. 2004. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 49: 243–270.
32. G. Loconsole, D. Cornara, R. K. Yokomi, and A. De Stradis. 2014. Infectivity and Transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 107:1316-1319.
33. Cornara, D., D. Bosco, and A. Fereres. 2018. Philaenus spumarius: when an old acquaintance becomes a new threat to European agriculture. J. Pest Sci. 91:957–972.
34. Roberto, S. R., P. R. S. Farias, A. B. Filho. 2002. Geostical analysis of spatial dynamics of citrus variegated chlorossis [sic]. Fitopatol. Bras. Vol. 27 http://dx.doi.org/10.1590/S0100-41582002000600007
35. Hill, B. L. and A. H. Purcell. 199. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 87:1197–201.
36. Cornara, D., A. Sicard, A. R. Zeilinger, F. Porcelli, A. H. Purcell and R. P. P. Almeida. 20. Transmission of Xylella fastidiosa to grapevine by the meadow spittlebug. Phytopathology 106:1285-1290.
37. Lopes, S. A. 2019. Scion Substitution: A new strategy to control citrus variegated chlorosis disease. Plant Dis. 104:
38. History and Status of the Glassywinged Sharpshooter in California. UCNFA News https://ucanr.edu/sites/UCNFAnews/newsletters/Download_UCNFA_News_as_PDF53795.pdf
39. Perring, T. M., C. A. Farrar, M. J. Blua. 2001. Glassy-winged sharpshooter host impacts Pierce's disease in Temecula Valley vineyards. Calif. Agric.
40. Anonymous. 2020. Wine Industry Advisor (Internet). https://wineindustryadvisor.com/2020/06/02/pierces-disease-resistant-vines-now-reality [many Internet news releases on “Pierce’s disease resistant varieties”]
41. Moralejo, E., D. Borràs, M. Gomila, M. Montesinos, F. Adrover, et al. 2018. Insights into the epidemiology of Pierce's disease in vineyards of Mallorca, Spain. Plant Pathol. https://doi.org/10.1111/ppa.13076
42. Berisha, B., Y.D. Chen, G.Y. Zhang, B.Y. Xu and T.A. Chen. 1998. Isolation of Pierce’s disease bacteria from grapevines in Europe. Eur. J. Plant Pathol. 104:427–433. [symptoms of PD in cermjan region of Kosovo in mid-1990s.]
43. Purcell, A. H. 1997. A regional problem or a world threat? J. Plant Pathol. 79: 99-105.
44. Guo, Y.-R., Q.-D. Cao, Z.-S. Hong, et al. 2020. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research vol. 7, Article 11. https://link.springer.com/article/10.1186/s40779-020-00240-0 [available on Internet]
45. Das, M., T. S. Bhowmick, S. Ahern, R. Young, and C. Gonzales. 2015. Control of Pierce’s disease by phage. PLOS One 10(6): e0128902. https://doi.org/10.1371/journal.pone.0128902
46. Baccari, C., E. Antonova, and S. Lindow. 2015. Biological control of Pierce’s disease by an endophytic bacterium. Phytopathology Vol. 103 https://doi.org/10.1094/PHYTO-07-18-0245-FI







Nessun commento:
Posta un commento